Indication & Important Safety Info
Indication
Important Safety Information
Indication
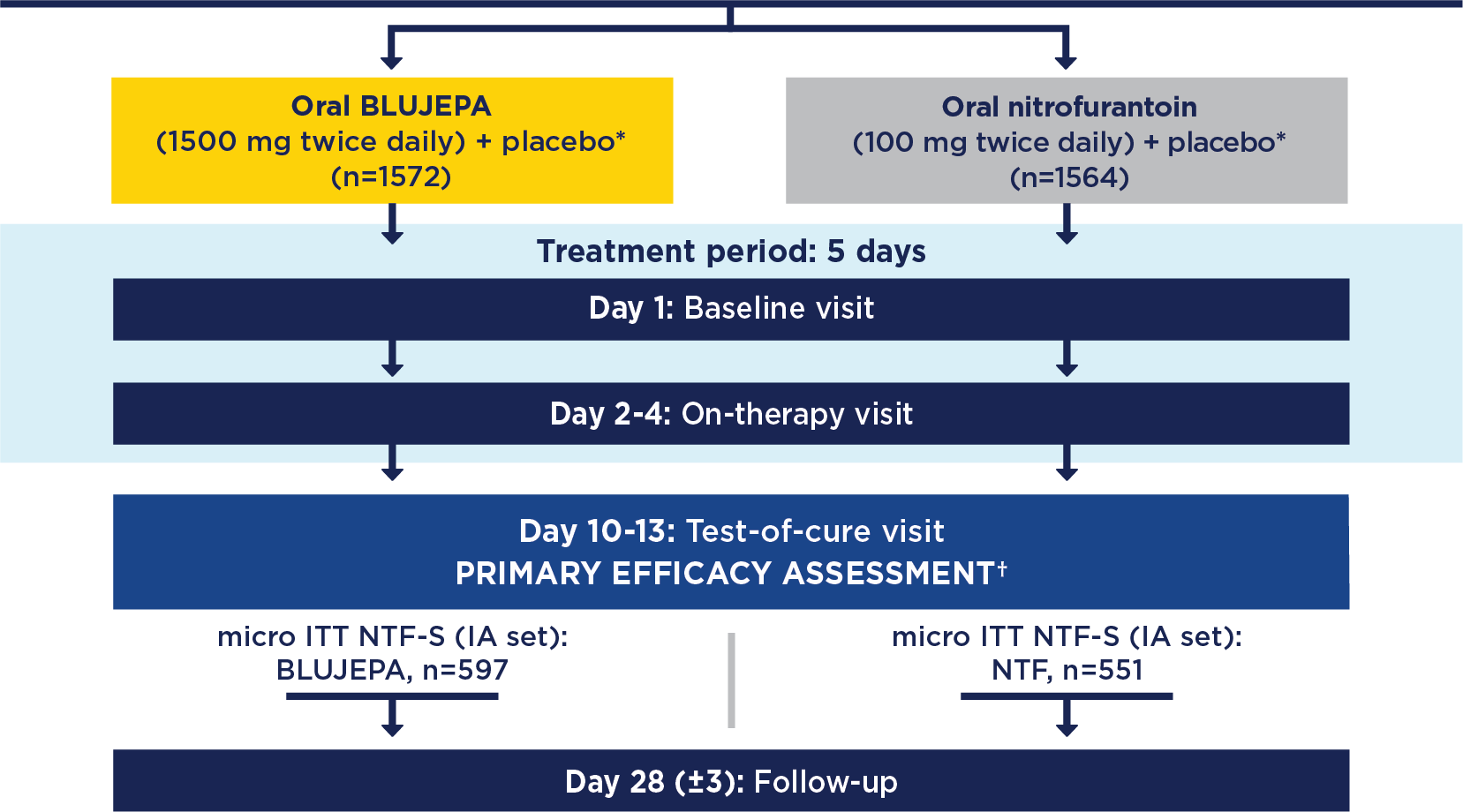
BLUJEPA is indicated for the treatment of female adult and pediatric patients 12 years of age and older weighing at least 40 kilograms (kg) with uncomplicated urinary tract infections (uUTI) caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus, and Enterococcus faecalis.
Usage to Reduce Development of Drug-Resistant Bacteria
To reduce the development of drug-resistant bacteria and maintain the effectiveness of BLUJEPA and other antibacterial drugs, BLUJEPA should be used only to treat infections that are proven or strongly suspected to be caused by bacteria.
Important Safety Information
CONTRAINDICATIONS
BLUJEPA is contraindicated in patients with a history of severe hypersensitivity to BLUJEPA.
WARNINGS AND PRECAUTIONS
QTc Prolongation
- A dose and concentration-dependent prolongation of the QTc interval has been observed with BLUJEPA. Avoid use of BLUJEPA in patients with a history of QTc prolongation or with relevant pre-existing cardiac disease, patients taking antiarrhythmic agents, and in patients receiving drugs that prolong the QT interval. Due to an increase in BLUJEPA exposure, avoid concomitant administration of BLUJEPA with strong CYP3A4 inhibitors, in patients with severe hepatic impairment (Child-Pugh Class C), and in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min).
Acetylcholinesterase Inhibition
- Dysarthria and other adverse reactions potentially attributed to acetylcholinesterase inhibition have been reported with BLUJEPA, an acetylcholinesterase inhibitor. Increased cholinergic effects can be associated with severe adverse effects, including atrioventricular block, bradycardia, bronchospasm, seizures/convulsions, and vasovagal syncope. Monitor patients with underlying medical conditions that may be exacerbated by acetylcholinesterase inhibition and patients receiving succinylcholine-type neuromuscular blocking agents, systemic anticholinergic medications, or non-depolarizing neuromuscular blocking agents.
Hypersensitivity Reactions
- Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving BLUJEPA. If an allergic reaction to BLUJEPA occurs, discontinue the drug and institute appropriate supportive measures.
Clostridioides difficile-Associated Diarrhea
- Clostridioides difficile infection (CDI) has been reported with nearly all systemic antibacterial agents, including BLUJEPA. Evaluate patients who develop diarrhea.
ADVERSE REACTIONS
- The most common adverse reactions occurring in ≥1% of patients are diarrhea, nausea, abdominal pain, flatulence, headache, soft feces, dizziness, vomiting, and vulvovaginal candidiasis.
DRUG INTERACTIONS
- CYP3A4 Inhibitors: Avoid coadministration of BLUJEPA with strong CYP3A4 inhibitors due to increased gepotidacin exposure.
- CYP3A4 Inducers: Avoid coadministration of BLUJEPA with strong CYP3A4 inducers due to decreased gepotidacin exposure.
- CYP3A4 Substrates: Avoid coadministration of BLUJEPA with drugs that are extensively metabolized by CYP3A4 and have a narrow therapeutic window.
- Digoxin: Due to an increase in digoxin exposures, consider monitoring digoxin serum concentration, as appropriate, with concomitant administration of BLUJEPA.
USE IN SPECIFIC POPULATIONS
- Renal Impairment: Avoid use of BLUJEPA in patients with severe renal impairment with eGFR <30 mL/min, including those receiving dialysis.
- Hepatic Impairment: Avoid use of BLUJEPA in patients with severe hepatic impairment (Child-Pugh Class C).
Please see accompanying Prescribing Information, including Medication Guide, for BLUJEPA.